Recombinant collagen
Recombinant protein is the application of recombinant DNA or recombinant RNA technology to obtain a recombinant
vector connected with gene fragments that can be translated into the target protein, and then transferred into the host
cell that can express the target protein, so as to obtain a protein with certain function and activity.
Recombinant proteins need to be prepared using an expression system, which can be obtained by in vitro method and
in vivo method. The production of recombinant proteins in vitro mainly includes four major systems: prokaryotic protein
expression (most commonly used E. coli protein expression), mammalian cell protein expression (commonly used cell CHO,
HEK293), eukaryotic expression system (yeast) and insect cell protein expression. The selection of the appropriate
expression system depends on the characteristics of the recombinant protein, the expected application of the recombinant
protein, and whether the system can produce sufficient amounts of protein. The appropriate protein expression system is
selected according to its downstream application to improve the expression success rate.
RGD targeted gene editing
At present, there are three kinds of expression systems that are most widely used, which are Esherichia coli(E. coli)
expression system, yeast expression system and CHO cell expression system. Among them, Escherichia coli(E. coli) is the
most commonly used host bacterium to express recombinant proteins, and has been used in the production of many
proteins with important medicinal value. Escherichia coli(E. coli) expression system has many advantages, such as fast
growth, low culture cost, clear genetic background and easy to achieve high density culture.
RGD is a tripeptide sequence containing arginine-glycine-aspartic acid, which is the recognition site of the interaction
between integrin and its ligand protein, mediates the adhesion between cells and extracellular matrix and intercellular, and
has the function of signal transduction, thus mediating many important life activities. In 1984, Pierschbacher and Ruoslahti
first described the RGD sequence as a highly conserved minimal integrin recognition sequence within fifienectin. RGD
exists in a variety of extracellular matrix, can bind to 11 kinds of integrins specifically, and can effectively promote cell
adhesion to biological materials.
By means of bioinformatics and computational simulation, we used collagen and fifiolin as templates to screen different
functional fragments, and combined with different structural functional areas (such as foldon area, adhesion promoting
peptide RGD, heparin binding area, etc.) for molecular design and splicing to construct a variety of artificial functional
proteins with different molecular weights. To obtain recombinant proteins with higher hydrophilicity, thermal stability,
proliferative activity and surface adhesion.
Advantages of RGD gene editing
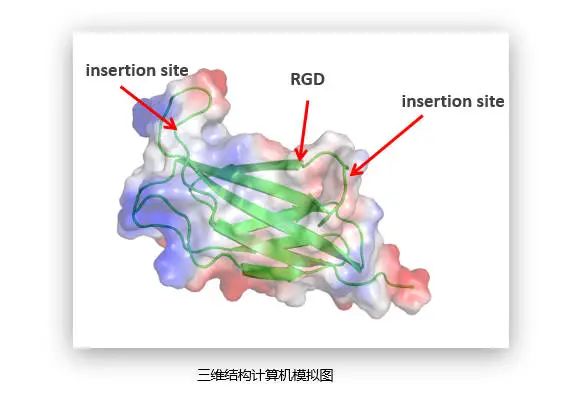
Computer simulation of three dimensional structure
Through computer simulation and molecular structure prediction, Recomtein AAG analyzed the three-dimensional spatial structure and key functional areas of collagen and fibronectin, intercepted functional active fragments of type I collagen, introduced RGD adhesion polypeptide in the N-end of the fragment, and made 8 replicates. Among the recombinant proteins, the structure of collagen is very special, it is a flexible fragment, while the RGD peptide can form a loop ring, which plays a good role in the middle of the two repeats. On the other hand, RGD peptide itself has a strong adhesion effect, and there is a synergistic effect between collagen and RGD, giving Recomtein AAG better activity.