Collagen and recombinant collagen
Collagen is widely found in skin, bone, muscle, cartilage, joint, hair, internal organs and other tissues of the human body, accounting for more than 30% of the total protein of the human body. Its main role is to maintain the shape and structure of skin and tissues and organs, and it is also an important raw material for repairing damaged tissues. It is called the life support of the human body. According to the specific amino acid sequence, collagen can be divided into 28 kinds, the common types are type I, type II, type III, type V and XI type, of which type Ⅰ, Ⅱ, Ⅲ collagen accounted for more than 90% of the total collagen.
70% of human skin is composed of collagen, so the collagen in the skin is closely related to skin aging. In recent years, collagen has become an emerging raw material in the field of cosmetics because of its tissue repair function, which has a good application prospect in anti-aging and repair of cosmetics.
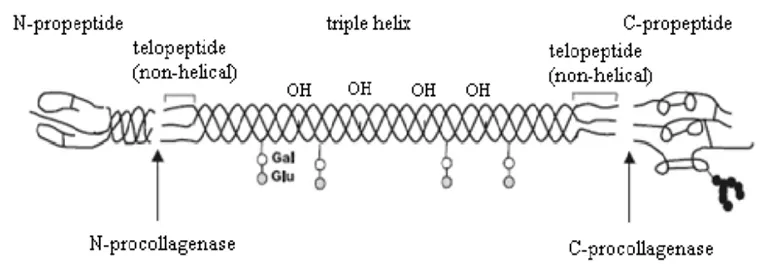
Figure 1 tripeptide chain structure of collagen
Recombinant collagen refers to the full-length or partial amino acid sequence fragments encoded by the specific type of human collagen gene prepared by genetic engineering technology, or the combination of functional fragments containing human collagen, and the amino acid sequence can be designed and improved according to the demand.
Skin care efficacy of collagen
Because of its excellent biocompatibility and low antigenicity, collagen has become a hot topic in the research of cosmetics, and its application in cosmetics is increasingly common. The main effects of collagen used in cosmetics are as follows:
① Nourishment: Collagen can enter the deep skin, give the skin the necessary nutrients, maintain the stability and integrity of the collagen fiber structure, enhance the activity of collagen in the skin, improve the living environment of skin cells, and promote the metabolism of the tissue, to achieve the purpose of nutrition and moisture.
②Moisturizing: Collagen contains a large number of glycine, hydroxyproline, hydroxylysine and other natural moisturizing factors, which are important substances to maintain skin moisture. There are a large number of hydrophilic groups such as hydroxyl and carboxylic groups on the outside of collagen molecules, so that collagen molecules can easily form hydrogen bonds with water, improve the skin's water storage capacity, and supplement the loss of collagen in the human body, so that the skin has a good affinity.
③ Repair: Collagen is similar in structure to collagen in skin, so it has excellent biological characteristics. Collagen can promote the proliferation and repair of epithelial cells, skin has a good absorption of collagen, supplement the necessary amino acids, so that the damaged and aging skin can be filled and repaired.
④Anti-wrinkle: The similarity of collagen and the structure of the skin stratum corneum determines its good compatibility, good affinity and permeability with the skin. It can penetrate into the epidermal layer of the skin, be fully absorbed by the skin, and form an extremely thin film on the surface of the skin, thus making the skin plump and wrinkles stretch, while improving the skin density, generating tension and having anti-wrinkle effect.
Method of obtaining collagen
1. Traditional collagen
Traditional collagen is extracted from the connective tissue of animals, and the extraction methods mainly include three kinds of physical methods assisted by high pressure, chemical methods of solvent extraction and enzymatic biochemical methods. Animal origin collagen has the characteristics of good film formation and skin affinity, so it has good moisturizing, repairing, nourishing and other functions.] However, due to the immunogen problems of animal-derived collagen and the manufacturing processes such as heating, physical shearing and even irradiation often used in the production of cosmetics, it is easy to cause degeneration and deterioration of animal-derived collagen and other stability problems. However, the use of plant-derived collagen is often limited due to its high molecular weight and unstable source, so manufacturers and researchers have paid more attention to recombinant humanoid collagen.
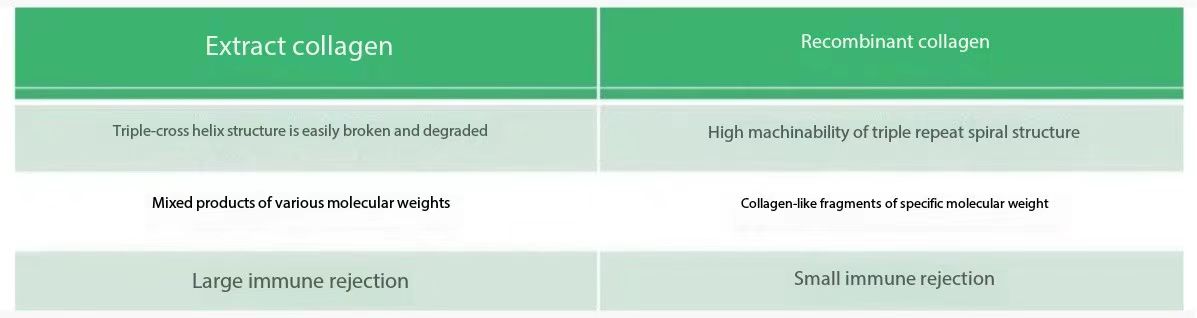
2. Restructure collagen
At present, many recombinant expression systems have been applied to recombinant expression of collagen, such as E. coli, yeast, animal cells, transgenic animals and transgenic plants, etc., as shown in Table 1.
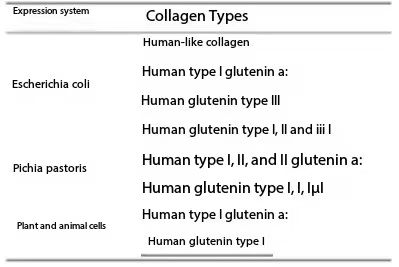
Table 1 Expression of recombinant collagen in each expression system
Due to the high cost, difficulty of culture, low expression level of recombinant collagen expressed by animals and plants, and in the laboratory stage, the current industrial production is still mainly based on Escherichia coli and yeast fermentation.
E. coli: pH value, temperature, dissolved oxygen, glucose and nitrogen are important factors that affect the expression of recombinant collagen and the growth of bacteria. Acetic acid is a byproduct of the fermentation process of E. coli and inhibits cell growth and collagen production. Carbon dioxide can inhibit or promote the growth and production of microbial cells, and is related to dissolved oxygen.
Yeast: Yeast has a strong preference for aerobic growth, cell density culture can be carried out, and the fermentation products are non-toxic side effects. However, insufficient or excessive methanol flow will affect the expression level of foreign proteins in Pichia Pastoris. In addition, temperature, pH, dissolved oxygen and other factors affect the expression level of collagen.
The spatial structure of recombinant protein can be solved by co-expression of proline hydroxylase or redesign of metabolic pathway.
The activity of collagen is highly related to the triple helix structure, the most important is the hydrogen bond between the three α chains, and the hydroxyl group of hydroxyproline can help form the hydrogen bond and improve the hydrophilicity of collagen.
Recomtein® HLC-Pro development features
Based on the disadvantages of traditional collagen extraction and the advantages of genetically engineered recombinant protein, we have developed a human-like collagen product Recomtein® HLC-Pro.
We analyzed the functional domain of collagen through AI big data, and screened the fragments with high activity, high efficacy and high safety. Then, exogenous genes were fused into the RGD binding site of collagen fragments, and their characterization and fusion effect were analyzed by three-dimensional structure AI computer simulation map, so as to evaluate their performance and safety.
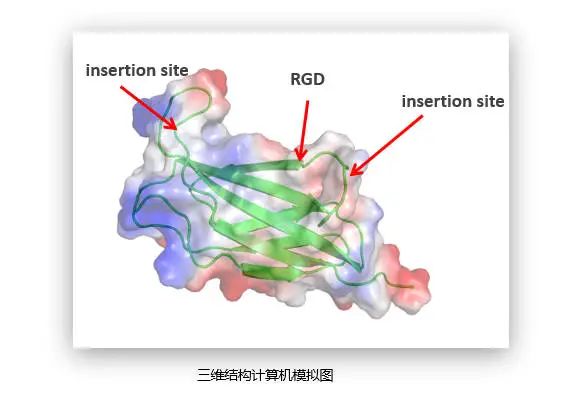
RGD, as an important recognition site for a variety of integrins, widely exists in the cell adhesion motif and is an important key site for cell adhesion and migration.
Advantages of RGD targeted gene editing
Improve the activity of targeted protein
Improve the purity of targeted protein
Promote cell adhesion to targeted proteins
Enhance the affinity of the targeted protein to the cell
Improve the absorbability of the targeted protein
Improve the stability of the targeted protein
Structural stability
Functional stability
Environmental stability
Enhanced safety of the target protein
Good biological compatibility
It is safe for consumers and reduces the immune rejection of human body
Safe for the environment
Recomtein®AAG
1. Innovation

2. Won the 2018 "Top Ten Innovative Raw Materials Award"

3. Efficacy of Recomtein®AAG
①Promote collagen synthesis
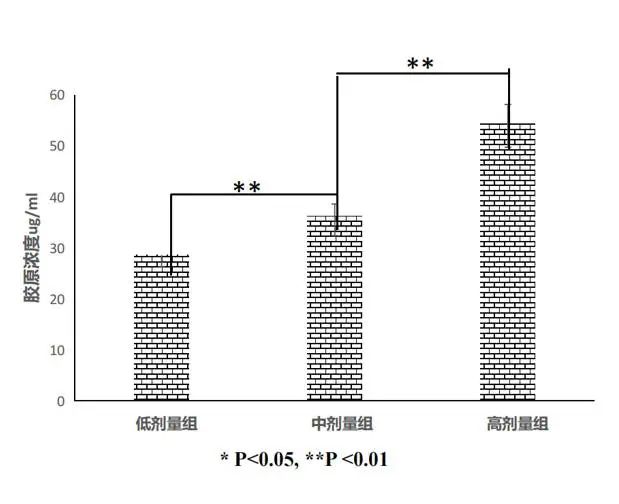
The results showed that as the dose of Recomtein®AAG increased, the ability to promote collagen gradually increased, thus better promoting skin remodeling, enhancing elasticity, and enhancing the effect of tightening the skin.
②Promote cell adhesion
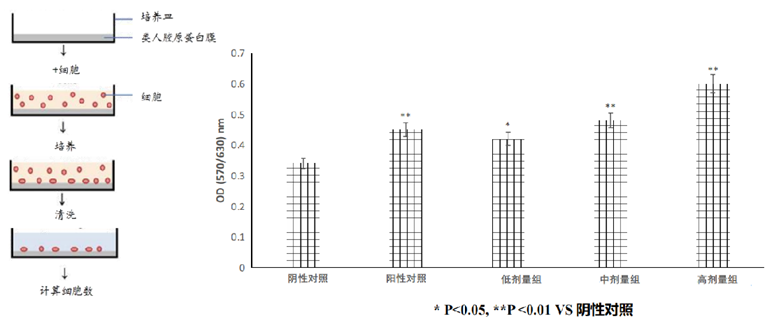
Recomtein®AAG has been shown to be excellent for repairing skin texture and improving skin barrier by promoting cell adhesion, enhancing cell growth and accelerating cell metabolism, thus efficiently promoting wound healing and collagen synthesis.
③Moisturizing and anti-aging properties
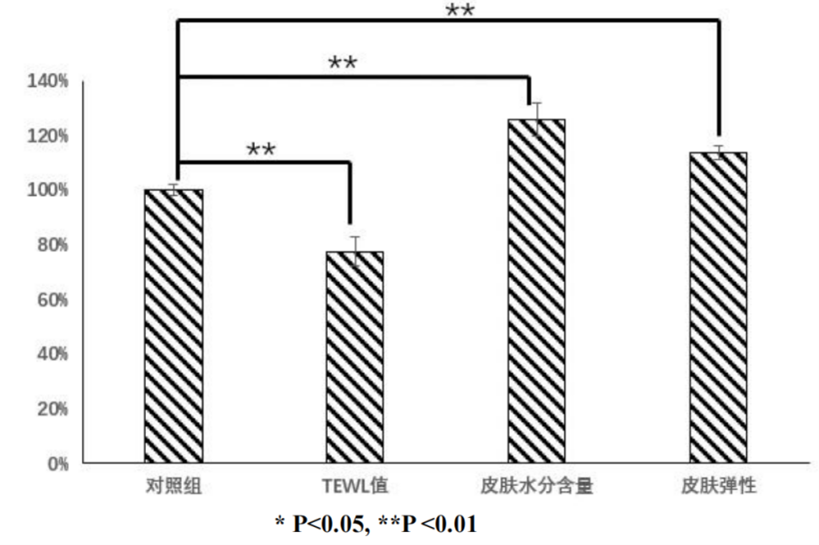
The results found: After 8 weeks of use of Recomtein®AAG serum containing 400ppm, the TEWL value of the skin was reduced by 22.36%; Increased skin water content by 25.87 percent; Skin elasticity increased by 13.68%; And brings significant barrier repair effect to the skin.
④Anti-redness effect
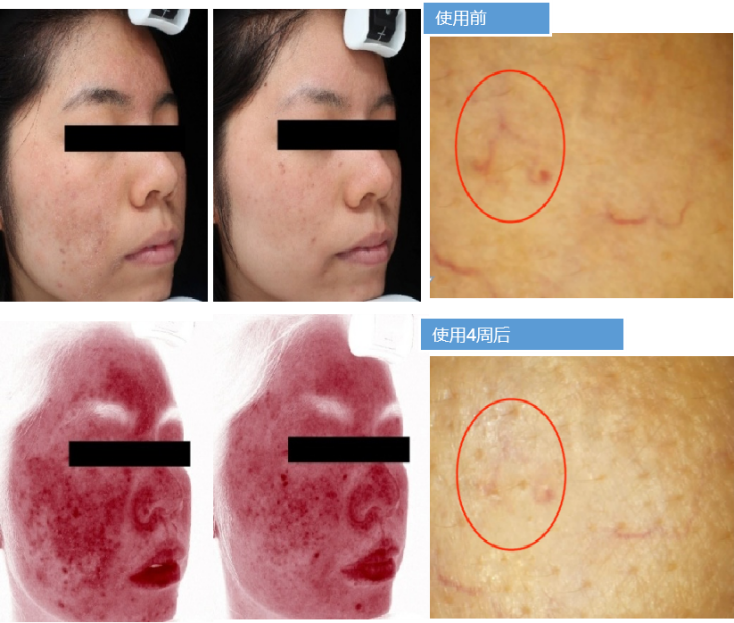
使用Recomtein®AAG has been used for 4 weeks to significantly improve the redness of the skin, indicating that Recomtein® AAG has the effect of removing redness and repairing sensitive skin.
⑤Anti-wrinkle effect
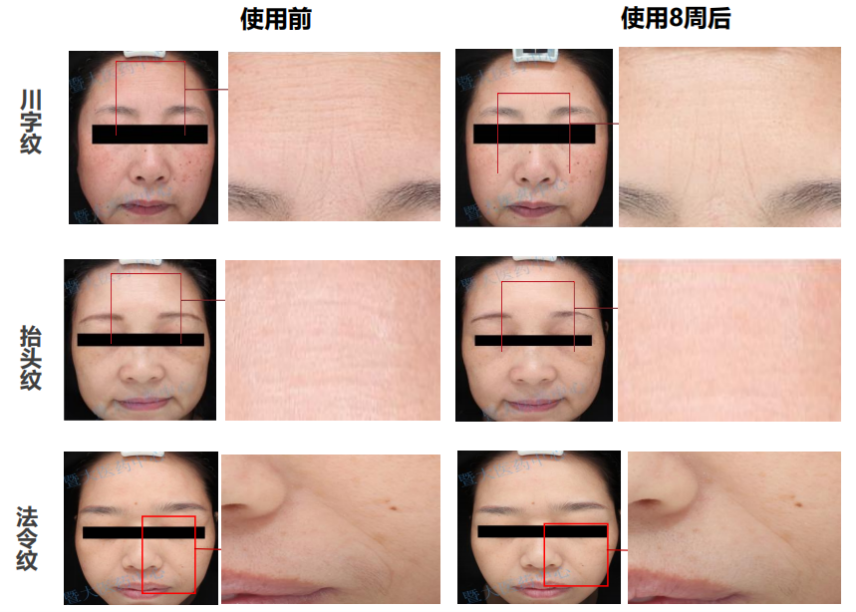
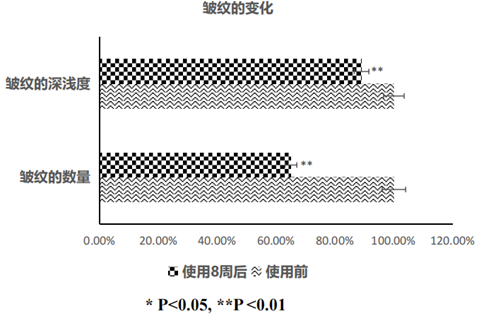
Compared with 8 weeks after use, the number and depth of skin wrinkles are significantly improved, the number of wrinkles is reduced by 35% on average, and the depth is lightened by 11% on average, which has excellent wrinkle-removing effect and brings elastic luster to the skin.
References
[1] Wang Xiaojun. Research on Application of Optimal dosage form of Humanoid collagen Series cosmetics [D]. Northwest University,2019.
[2] ZHANG H. Expression and purification of recombinant humanoid collagen and its application in cosmetics [D]. Jinan University,2017.
[3] Wang Yulin. Research progress of collagen application in cosmetics [J]. Gelatin Science and Technology,2012,32(01):8-12.
[4] LIANG Meizhen. Application of protein in cosmetics [J]. Guangdong Chemical Industry,2009,36(12):104-105.
[5] Shi Changsong, Cui Fengling, Zhang Hongguang, et al. [5] Shi Changsong, Cui Fengling, ZHANG Hongguang, et al. Study on moisture
absorption properties of common moisturizers used in cosmetics [J]. Journal of Household Chemicals,2007,30(1):25-30
[6] ZhA Qing-Qing, Nie Shan-Shan, Yu Wen. Research progress and application of recombinant collagen [J]. China Detergent Industry,
2022(08):41-45.
[7] TANG Yunping, ZHENG Qiang, Hu Bin, CAI Jin, Huang Lei, XU Zhinan. Research progress on preparation and application of recombinant
collagen [J]. Science and Technology of Food Industry,2016,37(18):384-386.
[8] Pre-culture of mesenchymal stem cells within RGD-modified hyaluronic acid hydrogel improves their resilience to ischaemic conditions
[9] RGD modified polymers: biomaterials for stimulated cell adhesion and beyond
[10] Cell adhesion and proliferation on RGD-modified recombinant spider silk proteins
[11] p48.2蛋白中RGD结构域对其生物学活性的影响及p48.2蛋白多克隆抗体的制备
[12] Integrin binding human antibody constant domains—Probing the C-terminal structural loops for grafting the RGD motif